Decoding the Enigma of Mushrooms: The Future of Superfoods and Scientific Discovery
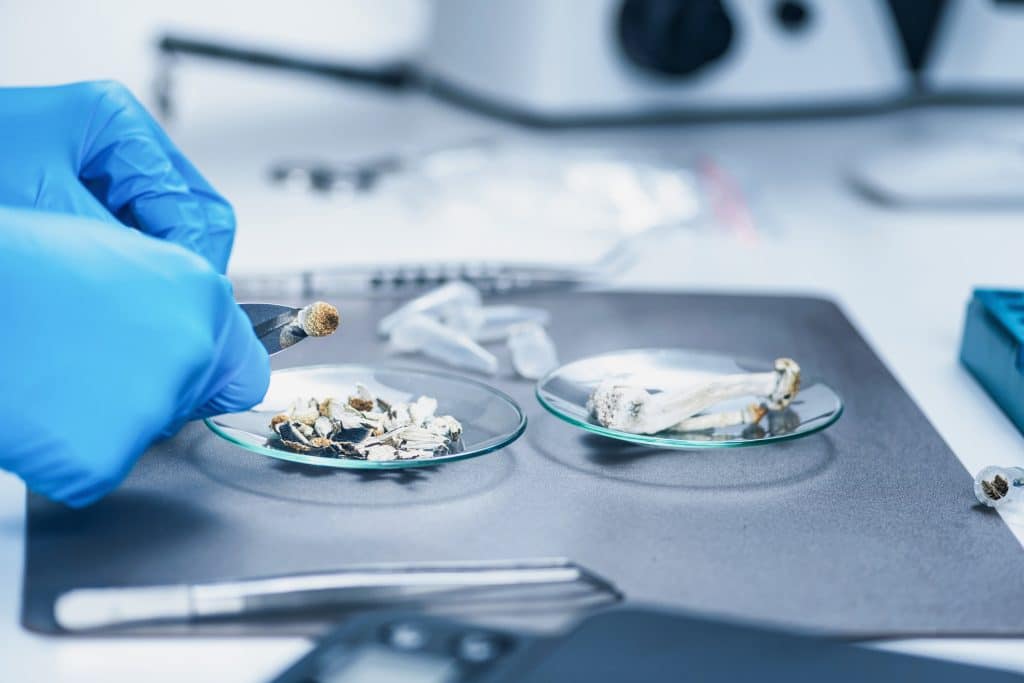
In an era where the fusion of ancient wisdom and modern scientific discovery shapes our understanding of nutrition, mushrooms emerge as a compelling narrative in the search for superfoods. At ADACT Medical, our mission to lead in analysis, testing, and compliance across health sciences finds a vibrant illustration in the study of mushrooms. This blog […]
Streamline Your Compliance: Join ADACT’s Primary Authority Partnership

Are compliance inspections causing you a headache? With ADACT’s Primary Authority Partnership, you gain government-backed support to overcome the complexities of TPD/TRPR and GPSR regulations, saving you time and protecting your revenue. Unlock These Exclusive Benefits: Expert Advocacy: Peterborough & Cambridgeshire Trading Standards will guide inspections, letting you focus on growth. Tailored Advice: Receive authoritative, […]
New Zealand smoking ban scrapped

New Zealand smoking ban scrapped The New Zealand has scrapped its smoking ban, its ground-breaking law that aimed to create a smoke-free generation. The decision, made by the country’s new National party-led government, has drawn widespread criticism from health experts and advocacy groups. The law inspired a similar policy in the UK, and is designed […]
Vape pods require 2ml fill certification

Vape pods require 2ml fill certification With the future of disposable vapes (aka disposables) in doubt, pod systems or pods, are back in vogue. It is important to highlight that pods fall under the same 2ml fill regulations as disposables. Tobacco and Related Products Regulations 2016 (TRPR) for e-cigarette tanks The Tobacco and Related […]
Do you know your 10ml packaging regs? There is a common mistake people are making…

10ml packaging regs As 10ml bottle of e-liquids sales start to climb here in the UK, we have seen clients refreshing their 10ml ranges, and in some cases re-launching them after some time off the market. But there are some anomalies to the 10ml packaging regulations. One common mistake that we are seeing, is placing […]
Black cohosh testing

Black cohosh testing ADACT Medical has recently conducted black cohosh (cimicifuga racemosa) testing for the Daily Mail newspaper. An in-depth investigation published by the UK’s Daily Mail Online on 13 November 2023, highlighted that popular herbal menopause remedies containing black cohosh – and promoted on Amazon and Google – have very low (or dangerously high) […]
What does the future hold for the vaping industry?

Damien Bové, ADACT’s Chief Regulatory Officer & Scientific Advisor discusses the future of vaping There has been chatter in the vaping industry about a possible ban on disposables, but is this just the start of a challenging time for the vape industry and what we need to be thinking about over the next few months […]
How is CBD testing carried out?

How is CBD testing carried out? ADACT Medical offers specialised CBD testing for a range of finished and bulk products that provide fast, accredited and accurate results. Our testing can determine CBD content, cannabinoids profile, purity, and product stability. We offer accurate, efficient testing with a five to ten working day turnaround as standard. […]
Reasons why you should call ADACT

Reasons to call our friendly, expert team Founded in 2012, ADACT Medical is a leading regulatory compliance specialist with cutting edge analytical testing services: 1. ADACT were pioneers in the implementation of the Tobacco Products Regulations (TPD) in 2016 and have helped hundreds of businesses and entrepreneurs achieve compliance. 2. We have a state-of-the-art ISO/IEC […]
As disposable vapes face a possible ban in the UK, pod systems are back in vogue

Damien Bové, ADACT’s Chief Regulatory Officer & Scientific Advisor discusses a possible ban of disposable vapes As widely discussed in the UK press and media, a disposable vapes (single use vapes) ban is on the horizon, but this is not an unexpected move and many companies have been preparing by updating their pod systems. These […]
